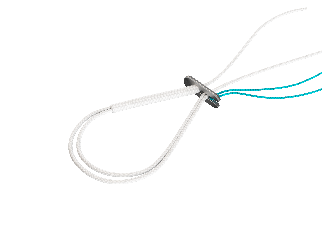




COMETE Control and Extraplate
COMETE Control is an adjustable cortical fixation
- Loop length adjustment
- Loop locking
COMETE Extraplate
- Plate dedicated to COMETE and COMETE CONTROL
- Dedicated to 6 to 11 mm tunnel
 |
COMETE Control The high-strength fastener: - Pull-out strength 1844 N*. Cortical support: Titanium plate for 4.5 and 5 mm tunnels:
Graft held in place by a wide braid - Polyethylene braid: Ø 1.35 mm Manual locking |
.png) |
COMETE Extraplate On flush-mounted plates: triples the contact surface, increases thickness by 0.5 mm Dimensions
|
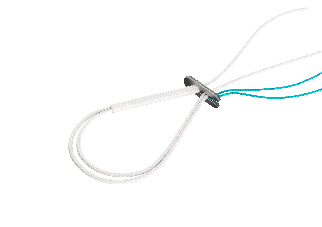 |
COMETE Control + COMETE Extraplate The adjustable fastener for tunnels from 6 to 11 mm |
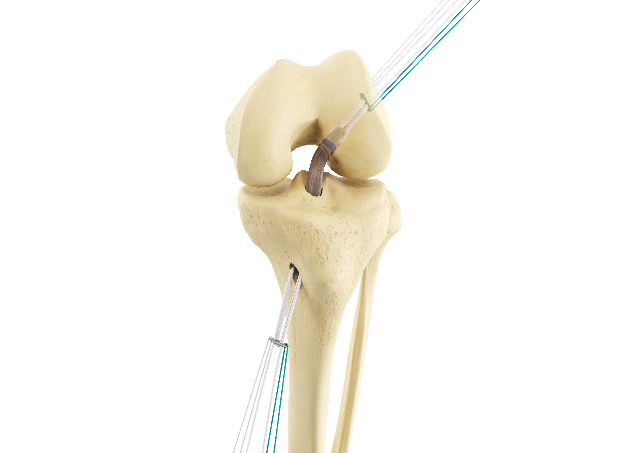  |
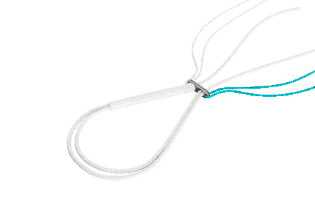
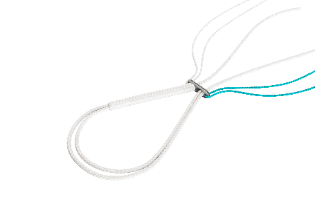
Adjusting and locking the adjustable buckle : Adjustment: alternately pull the white braid and the braid with the black end until the desired setting is reached. Locking: hold the white braid taut and pull the braid firmly with the black end. This action locks the adjustable buckle. |
* Result obtained after dynamic test (device only) carried out as part of the CE marking of the product.
** Diameter measured under load
The choice of fixing for your DIDT / DT3 and DT4
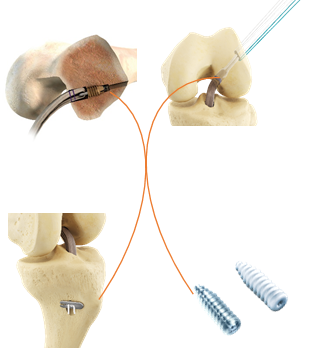 |
Femoral fixation COMETE Control ACLip COMETE Control ACLip |
Tibial fixation COMETE Control COMETE Control Vis ECLIPSE BCP or Profil VIS ECLIPSE BCP or Profil |
COMETE Control range:
| Designation | Reference | Implant |
| COMETE Control | OAMGEFCR1U |  |
| COMETE Extraplate | OAMGEPLA1U |  |
Download documentation
Download the documentation
Creation date : January 2021 - Manufacturer : COUSIN BIOTECH - Product name : COMETE Contrôl - Intended use: ligament or tendon anchoring device for orthopaedic surgery, in particular for reconstruction of the cruciate ligament of the knee. DM class: IIb - Reimbursable by health insurance organisations in certain situations: consult the terms and conditions on the ameli.fr website - Indications and recommendations for use: the instructions on the product labels and instructions for use should be read carefully.
Cousin Biotech S.A.S with capital of : 340 656 € - 398 460 261 RCS Lille - N°TVA FR 34 398 460 261
Internal reference: 19/03/AMPLITUDE/PM/001.




